By turning glucose, amino acids, and fatty acids from your diet into ATP, mitochondria generate energy. I’m aware that I’ve made this comparison in prior works, but I always think of mitochondria as little versions of Mighty Mouse from the beloved 1950s cartoon. When I was younger, I loved seeing Mighty Mouse “save the day” by using his amazing strength and invincibility. Like the mouse, your mitochondria may be little, but they are strong. By generating the energy your body requires to function, they also save the day.
Unless you’re a student studying for a biology exam, it’s likely that you don’t think about mitochondria very much these days. After all, they do silent and undetectable work. But trust me when I say it takes a herculean effort to make all that ATP. The energy needs of the human body are astounding. It is believed that an average-sized individual in good health produces about 140 pounds of ATP each day2. Yes, you read that correctly pounds. Considered as a very conservative estimate, you probably consume around 3.5 pounds of food per day. This is a fantastic return on investment. (If you’re wondering, “Wait only weigh 140 pounds! How is that possible? Where does all that ATP go?” the answer is straightforward: your body’s cells used it. When you’re active, your cells’ energy demands are much, much higher.)
Is it any wonder that mitochondria are described as the cell’s powerhouse?
AN EVOLUTION IN THE PRODUCTION OF ENERGY
The history of mitochondria is intriguing. Their evolution from absorbed bacteria is the most widely accepted idea for how they came to be. There were many different kinds of bacteria in the Earth two billion years ago, but there were also some newly emerging cell forms in the mix. According to the legend, one of those cells, which was probably an ancestor of the eukaryotic cells that make up the majority of life on Earth, absorbed a particular bacterium. They began collaborating, creating a mutually beneficial connection known as symbiosis. The bacterium aided the cell’s ability to respire that is, use oxygen to produce energy. The bacterium received a home from the cell in exchange, which helped to protect it from the environment. The bacteria that lived inside of those cells transformed into mitochondria over millions of years.
Although mitochondria are found inside human cells, they have never completely shed their bacterial roots; in fact, they resemble the gut bacteria that make up your microbiome. In reality, mitochondria have their own DNA, exactly like your “gut buddies,” as I like to call them. They can divide concurrently with the division of their host cells, but they can also divide at any moment to produce additional mitochondria through a process known as mitogenesis. You will soon learn how important it is for you, your health, and your fate that they can reproduce without the rest of the cell having to divide.
Your mitochondria and microbiota are still connected today due to their similar bacterial ancestry. Postbiotics are signaling molecules that keep them in touch. Although many of them are also found in some foods we eat, postbiotics are mostly created by the microorganisms in your gut. Your body’s activities are closely watched by the bacteria in your gut. They’re in a wonderful position to perform the task because they routinely acquire data about the situation from both your neurological system and immune system. The amount of energy required is then communicated to the mitochondria via postbiotic signaling molecules. These messages range from “Hey, let’s rev up production, we have a huge exercise today!to “Oh no! We may have consumed a contaminated meal. The information that your mitochondria receive from your microbiota has a significant impact on how much energy is being produced by your mitochondria. Let’s shut everything down till we figure out what’s going on. Another reason why typical ketogenic diets, which limit plant-based fiber, can result in negative side effects like weariness and mental fog, is because of this. A healthy microbiome requires that fiber to thrive. In turn, a healthy microbiome generates postbiotics. We’ll talk about this in more depth shortly, but let’s look at our energy factory in more detail first.
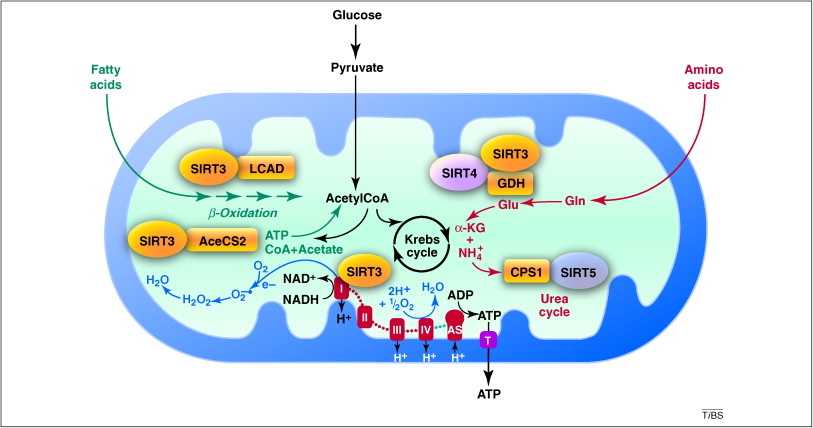
MITOCHONDRIA’S ENERGY-MAKING PROCESS
Cellular respiration is the scientific term for the process through which food and oxygen are converted into energy. Every single mitochondrion in the body engages in this process repeatedly, and as you may recall, your body has trillions of trillions of mitochondria. Cellular respiration can be compared to an internal production line. The process of turning glucose into ATP entails several phases.
Humans are carbon-based living forms, as any Star Trek fan will tell you. Additionally, we use carbon. Whether our meal is composed of fats, carbohydrates, or amino acids, all of it eventually decomposes into a large number of carbon molecules. These carbon molecules enter the cells, where the mitochondria take them up to begin the energy-production process. The Krebs cycle, also known as the citric acid cycle, is where the cascade of events that converts those carbon molecules into ATP begins. (Aside: Drs. Veech and Cahill were mentored by Hans Krebs, the scientist who originally characterized this cycle and received the Nobel Prize for his research.
After entering the mitochondria, the carbon molecules start a fascinating dance with protons and electrons, some of which originate from water. You might remember that these particles are chargedâthink of them as electrified. The protons are positively charged (+), while the electrons are negatively charged (). The inner mitochondrial membrane of the organelle is then crossed, allowing the protons and electrons to enter. The electron transport chain is a series of chemical events that take place there. It’s complicated, but to put it simply, that chain aids in increasing the charges of those particles. The protons and electrons that are excited as the charge rises turn into the classic “hot potatoes,” getting hotter and hotter as they jump from one level of charge to another.
This process can be compared to a group of twentysomethings leaving for an evening at the hottest new nightclub because things are so “hot” right now. Consider one mitochondrion as the newest and trendiest club in town. It will be known as the Mito Club. Customers can enter this trendy establishment using a single main door and leave through a back one-way revolving door. The Mito Club does wish to stay in code, after all, but we’ll discuss those later. At this point, there’s only one route in and one way out for the Mito Club’s patrons.
There are hundreds of protons, electrons, and other molecules like oxygen and hydrogen crammed into the sweltering, congested Mito Club. A doorman watches the entry because the Mito Club can get so crowded everyone who is somebody is trying to get in. His responsibility is to limit admissions. However, despite the doorman’s best efforts, it’s difficult for customers to enter the bar without running into at least a dozen other individuals. A lot of protons and electrons are present with the intention of interacting with an oxygen molecule, much like at a genuine club.
Some are successful in coupling with the desired oxygen. They join arms and walk toward the back rotating door, making a lot of ATP as they leave. It resembles the process by which water drives a mill wheel to produce power. Together with oxygen, the positively charged protons create some of the crucial energy currency as they move through the mitochondrial membrane. (Furthermore, the protons leave carbon dioxide, or CO2, behind when they leave. In this scenario, think of CO2 as the beer bottles and other rubbish the proton customers are throwing away before leaving the establishment with their dates.)
A large number of the electrons suddenly decide they’ve had enough and are leaving on a usual busy Saturday night. They were seduced by the prospect of having fun somewhere else, perhaps with an oxygen molecule or two. This leaves a number of protons, who had also hoped to connect with some oxygen, loitering around and recognizing that their chances of doing so are rapidly dwindling. The irate proton now rushes for the door after spotting the exit sign in the distance.
Some of those protons may ultimately pair up at the exit when they run into some stray oxygen molecules. The high-energy molecule ATP is then created as these fresh couples try to squeeze through the rotating door. Most of the other protons won’t have as much luck, though. They will leave the club alone and empty-handed. No ATP will be produced tonight.
I should point out that this procedure isn’t quite as straightforward as I just said. Keep in mind that the Mito Club is warm due to the collisions between protons, electrons, and other molecules. As you may expect, the circumstances can cause some shoving and pushing. When the doorman notices problems developing, he will send his bouncers to handle the situation so that the protons can couple with oxygen peacefully. In genuine mitochondria, this complex process of attempting to mix oxygen with protons to form ATP can result in more than just a small amount of CO2 being produced. Reactive oxygen species (ROSs), which are potentially dangerous pollutants, can be produced as a result of the interaction of these various particles and molecules. Imagine ROSs as being somewhat similar to the exhaust from your car. They represent the customers who have overindulged in the Mito Club simile and start punching one other. Even though the bouncers will eventually deal with them, they nonetheless make a scene.
See, occasionally ROSs, which include the free radicals that we health experts talk about so much, are formed when electrons wind up connecting with oxygen instead of protons. By causing oxidative stress, these ROS harm the mitochondria and consequently the cell. Most likely, you’ve heard about ROSs and oxidative stress. Both have been linked to chronic illness and aging.
Some ROSs are now acceptable. If there wasn’t some excitement, the Mito Club wouldn’t be the place to be. After all, the smell of danger can be rather enticing. They function as signaling molecules in modest concentrations, transmitting messages to support the health of your cells. Only when ROS production is excessive do they pose a threat. When oxygen molecules and too many electrons pair together, your mitochondria may be harmed. Even worse, the bouncers of the Mito Club may be able to cause apoptosis, which is the cell’s explosive and quick demise, if they can’t discover a means to control the ROSs. If there were too many altercations and turmoil, the club would have to close.
Melatonin, the sleep hormone and antioxidant you’ve heard so much about, and glutathione, a less well-known but equally important antioxidant, are the two main bouncers at the Mito Club. They support the maintenance of those ROSs in their optimal concentration, which is precisely the right amount for them to carry out their signaling functions without endangering the cell. As you can expect, the Mito Club prefers to have a large number of those bouncers on hand to ensure that things don’t get out of control. (You’ll discover that, contrary to what you might believe, you really have greater control over ROS generation in the next chapters.)
WHAT ABOUT KETONES?
The electron transport chain (ETC) and the Krebs cycle are briefly summarized using the analogy of a club. The mitochondria combine chemicals from the food you eat and the oxygen you breathe as they enter your cells to create ATP. Sugars, amino acids (from proteins), and free fatty acids (from dietary fats) can all be converted into energy by mitochondria. A crucial component in the creation of this energy currency is oxygen. But because energy synthesis causes oxidative stress, it also has the ability to harm, if not completely destroy, mitochondria. Your mitochondria change to a sluggish burn at night for this reason. They take advantage of the slower production times to relax and perform any essential maintenance. The need of obtaining enough sleep for maintaining mitochondrial function is another reason why so many people who have insomnia also tend to be overweight.
Think at it like this: if the club opens at six and closes at two every night, there is plenty of time to clean the place up. 16 hours are allotted for cleaning up spilt beverages, trash, and other debris that the ROSs left behind. Imagine that the club’s hours are extended until four in the morning; the cleaning staff would only have six hours to finish up! The crew will have to make some compromises because of time constraints. Garbage will begin to build up, and the floor will still be a little bit sticky. The Mito Club is going to quickly turn into a seedy location where nobody wants to go. Your mitochondria are affected by a shorter sleep cycle in the same way. They won’t have enough time to tidy up, fix any harm, and get back to fully functional!
Do you know what prompts this necessary downtime for repairs? the emergence of ketone bodies and free fatty acids. That’s correct, the mitochondria change when there is no new food to digest. When it’s time to clean up the Mito Club, they begin using the fat that has accumulated in your fat cells as a signal. This fat was created from all the excess sugar, protein, and fat that you didn’t use during the day. These fats are kept until they are released as free fatty acids into the circulation when food consumption stops. They can be compared to your slow-burn energy.
Your mitochondria, especially those found in your brain cells, have the ability to utilize ketones as fuel under specific circumstances. In general, this happens when sugar supplies are low for an extended period of time. This might be the case if you’ve been on a ketogenic diet, which limits your intake of carbohydrates and proteins, have fasted for about 12 hours, have engaged in some extremely strenuous exercise, have burned through all of your glycogen stores, or, in the worst-case scenario, are actually starving. Just to be clear, ketones are essential for maintaining the health of the mitochondria. But as we’ve spoken about, people don’t really understand that function.
Our energy generation process resembles a hybrid car in certain respects. The battery is recharged while the automobile is operating on gasoline (glucose), and this stored energy can be used after the gas has been used or the engine has been shut off completely. The mitochondria use this battery power, in the form of free fatty acids or ketones, at night after you haven’t eaten for at least eight hours to produce the ATP you require.
AUDIENCE CONTROL
The Mito Club can only accommodate a specific number of guests at once. After all, there is the fire code to take into account. But occasionally, the establishment is visited by more people than expected. like after a substantial dinner. The club, which stands in for one of your mighty mitochondria, struggles mightily to accommodate everyone who enters while also making every effort to allow protons and oxygen molecules to link together so that glucose can be converted into energy. Unfortunately, the Mito Club just cannot keep up with the public, especially with today’s average diets.
You probably have an idea of what will happen next. Energy production slows down while fat deposits increase when your mitochondria are forced to balance ATP generation with storage while also defending themselves from those possibly harmful ROSs.
Now, it wouldn’t be a major concern if the club only sometimes experienced overcrowding. After all, even our hunter-gatherer ancestors occasionally encountered a time of abundance and relished a lavish feast to mark the occasion. Your mitochondria are capable of handling occasional little amounts of excess. However, if that volume of participants lasts the entire day without a break, your mitochondria will suffer. There must be a better approach. If you will, the Mito Club’s backup plan.
At first glance, it might appear that the best course of action is to simply restrict the number of visitors allowed into the club. Perhaps the doorman should simply place one of those elegant velvet ropes in front of the entrance, allowing the mob to gather outside in a line. Although restricting access would seem like the logical solution, insulin, the hormone your pancreas produces to assist with carbohydrate metabolism, is not taken into account. Proteins and carbohydrates are diverted by insulin from the bloodstream and into the cells. It essentially bangs on the cell wall and inquires as to whether the cell desires or requires any protein or glucose.
If you stop eating, that velvet rope should provide your mitochondria the much-needed time they need to catch up on the backlog. Your pancreas will continue to release insulin as more and more digested food enters your bloodstream, building an army of insulin molecules to signal your cells to open up and let in the customers who are there to couple. But after all the banging, the Mito Club doorman is not going to give in. The final outcome? Less energy will enter the cells, energy synthesis will dramatically slow down, and blood sugar reserves will increase. Insulin levels will increase in an effort to fix the issue, but the line outside the club door will lengthen and become more raucous.
This issue becomes worse over time. If you’ve read a few books on the ketogenic diet, you might be thinking, “Well, surely the FFAs from your fat cells, as well as ketones from your liver, can get into the cells without insulin’s help.” Fats and ketones don’t need insulin to enter the cells; they can restart energy production, and they’re already waiting to be used in all those storage spaces (your fat cells). The harsh gut-punch, if you’ll excuse the word, is that insulin tells your fat cells to hold onto their fat reserves.
When your ancestors were able to eat that recently killed bison or those recently discovered ripe berries, they needed their insulin levels to rise in order to help save those sugars and proteins by ushering them into fat cells, so there is some evolutionary rhyme and reason for this blocking action. So, during those times of abundance, they need any fat-liberating action to be suspended in order for the body to reserve those extra carbohydrates and proteins for a rainy day. It would be absurd to simultaneously have someone unpack the storage locker if you were trying to store fat.
Consider this. Typically, a high insulin level indicates that you have recently eaten. You have the calories you require to produce energy! If there is any surplus glucose, it should be saved rather than consumed. After all that, it can be summed up by saying that insulin prevents your body from releasing stored fat while also assisting you in processing carbohydrates.
If you have a flexible, healthy metabolism, your insulin levels start to drop as soon as you finish eating. Since insulin levels have dropped, FFAs can now go from your fat cells into the waiting arms of your mitochondria, which require fuel. To keep your brain satiated until your next meal, your liver will be instructed to produce a little amount of ketones. Sadly, a large majority of us are now metabolically rigid. We overeat, our insulin levels are constantly elevated, and as a result, we have an abundance of fat reserves but no mechanism for releasing them for use.
This is the reason why when you start a high-fat or ultra-low-carb ketogenic diet, your metabolism will immediately sputter to a halt. Your mental clarity is cloudy, your energy levels plummet, and you generally feel miserable. This condition, sometimes known as the “keto flu” or the “Atkins blues,” is brought on by elevated insulin levels that prevent fat release and, as a result, the creation of ketones. Even though the majority of ketogenic diets assert that cutting out carbohydrates will cause your body to begin producing ketones, this is, quite plainly, not possible if you have insulin resistance, which is why my patient Angy was never able to lose weight on the diet. Her insulin levels were just too high to permit the release of her fat stores, similar to the many other dieters who have encountered frustrating results with this diet.
That being said, it is still feasible to go from insulin resistance to fat liberation so that your body can utilise it as food. Soon I’ll show you how to do that. Here’s a hint: ketones’ communication with your mitochondria holds the key to unlocking the mystery.
NOW IS THE TIME TO DISBAND
While many people nowadays refer to ending a romantic engagement as “uncoupling,” mitochondria have their own method of separating the processes of producing energy (ATP) and burning fuel (metabolism). Discussing mitochondrial uncoupling will now follow. The keto code can only be broken by uncoupling, which has many, many more advantages than just weight loss.
Let me use the Mito Club analogy to describe how uncoupling functions. You are aware that this juke joint is the place to be, and the line to enter has only grown longer. As it becomes increasingly hot inside, protons eventually lose interest in joining with some oxygen and prefer to go on to a different location or call it a night. There is only that one exit at the back of the Mito Club. The crowd present makes it simple to bottleneck the door. The adjoining emergency escape is then forced open by someone. Bam! The customers rush out the side door, feeling so liberated by their newfound independence that they move down the block to try their luck “coupling” somewhere else.
Tension eases as a portion of the capacity becomes available, allowing customers to resume their enjoyment. In fact, there is now space for those protons and oxygen molecules to recouple. Furthermore, some of those people who were waiting outside can now be admitted by the doorman. But after only a short while, the club is once again full. What is the owner supposed to do? Customers inside the tightly packed space as well as those outside waiting to enter are not thrilled with him. He needs a new strategy.
It’s obvious that the cell needs a few more clubs (mitochondria) to keep up with demand given the large number of customers hanging around outside without much to do. We refer to this procedure as mitogenesis, or mitochondrial replication, within the cell.
Your cell may actually produce additional mitochondria to manage the burden in some cases (you may remember that mitochondria have their own DNA and can divide, when necessary, regardless of what the rest of the cell may be doing). There are only two strategies to increase your mitochondria, according to the majority of experts: fasting and exercise. I’m here to tell you that mitogenesis can also be induced in a number of other ways. With the appropriate signals, you can decrease your fat accumulation rather than increase it.
The club’s owner decides to construct further clubs. He has mastered the formula for achievement with the Mito Club. But he needs a bank loan to build those new venues. Where does he go to get money of such kind? He can use the fat reserves as the building blocks for the new clubs (mitochondria) and restart the production of all that uplifting energy.
You might be asking yourself, “But why would the fat stores distribute their money? You just informed me that insulin resistance is the main reason why most people are unable to remove stored fat. You’d be right to believe that something doesn’t add up. To open the side doors, encourage mitogenesis, and signal the opening of the fat storage, special proteins must be involved and stimulated by ketones.
DISCONCUSSION FOR HEALTH
The Buck Institute for Research on Aging’s physiologists David Nicholls, Vibeke Bernson, and Gillian Heaton revealed that mitochondria have built-in “emergency exits” for the many actors involved in the electron transport chain in 1978. Uncoupling proteins are in charge of regulating those exits.
UCP1 to UCP5 are the five uncoupling proteins that are currently recognized. They are all found inside the inner mitochondrial membrane and, under specific conditions, permit protons to leave. Our mitochondria can allow uncoupled protons to escape the cells’ energy-producing power plants, wasting calories in the process like Mito Club customers sneaking out the back door.
Ketones, as well as other substances that we will cover in later articles posted on a blog, signal the mitochondria to uncouple or open up those emergency exits, causing them to produce less ATP than they could otherwise. The mitochondria physically waste calories during this process as opposed to utilizing them as fuel.
You might be perplexed as to how creating ketones which will result in you waste perfectly good fuel could possibly be a good choice when your body perceives that it is starving. It is undoubtedly paradoxical. But as we’ll see shortly, our mitochondria “uncouple to survive.” As first explained by Martin Brand, PhD, a well-known researcher who focuses on the mechanisms of energy conversion in the human body, mitochondrial uncoupling is all about safeguarding the mitochondria themselves. Additionally, mitochondrial uncoupling generates heat through a process known as thermogenesis. That heat is important for promoting vigor, weight loss, and good health. This may help to explain why Drs. Veech and Cahill were so sure that ketones were some types of “miracle fuel.”
Are you prepared to begin? I’m going to show you how to take advantage of mitochondrial uncoupling to burn waste fuel and survive while doing it in the articles that follow. Your mitochondria will be released once they are unlocked, along with the additional pounds you’ve been attempting to shed.